

Loss of tissue in maxilliofacial region is so common due to various reasons like trauma, cancer surgeries etc…With the arrival of tissue engineering methods viable tissues can be “cultivated” in the lab and can be used for curing the defects, novel method of 3D bioprinting is the new hope in tissue engineering.
The ability to print biological tissues opposite
to traditional 3D plastic and metal printing
has resulted in the birth of the new bio printing
and Tissue Engineering research field. 3D bio
printing is a computer-aided deposition of cells,
biomaterials and biomolecules. The advantage
of 3D bio printing compared to traditional tissue
engineering is assembling cells, biomaterials and
biomolecules in a spatially controlled manner to
reproduce native tissue. In the future, due to highresolution characteristic of printing technology with novel printable biocompatible materials or ‘inks’,
autologous tissue will be 3D printed with macroand micro-architecture for reconstruction. The focus
is by controlling the micro and macrostructures to
replicate complex native-like tissue architecture
more reliably than by conventional methods. The
wide synergy of research on biomaterials and on
3D bio printing may enable restoring the form
and functional reconstruction of OMF anatomy
in the near future. 3D bio printing avoids donor
site complications and immunosuppression. The
main obstacles for wider use for 3D bio printing
are related to biology, technology and regulatory
issues.
Traditional 3D printing is relatively simple and
can be performed by the home computer using
the proper software. For medical use, 3D digital
data are acquired from computed tomography,
magnetic resonance imaging, or laser scanning.
These data can be manipulated by CAD–CAM
software and be converted into Stereolithographic
format for printing. Fabrication of solid bio model
is carried out under the computer guidance to accurately and in a controlled manner deposit
biological materials in a layer-by-layer fashion. 3D
bio printer uses a nozzle to deposit biomaterials
and cells according to xyz-axis to create the
structure required. Fabricated solid model is then
cultured in a bioreactor under specific conditions to
produce specific and designed tissue engineered
vital tissue.
Cells derived from patients preferably stem
cells are cultured in optimum conditions. These
cells are provided with adequate growth factors
enabling them to grow and multiply. When they have multiplied to enough numbers they are
collected formed into spheroids and loaded into
a cartridge to create a bio ink.
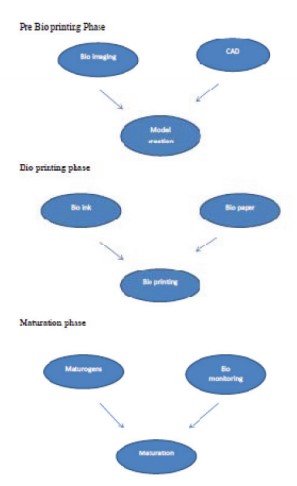
Bio printing involves three phases
Although anatomical and functional bone identical
to original jawbone cannot be produced at the
present, there is good evidence that Tissue
Engineered bone identical to missing bone part
will be available in the near future. This is an area
that could revolutionize the oral and maxillofacial
reconstruction in near future.