

Bioceramics are an important subset of biomaterials which act as an excellent bone substitute which is been used in many fields of medicine. Bioceramics range in their biocompatibility from the ceramic oxides, which are bioinert, to the other extreme of bioresorbable materials, which are eventually replaced by the body after they have assisted the repair of cells. Its application in dental implants has gained interest in the past two decades. This review is attempted to emphasize the approaches done in bioceramic materials for use in dental implants.
Key words: Bio ceramics, zirconium, bioimplants
The efforts to restore completely and partially
edentulous arches have been practiced by
clinicians since centuries. By the introduction of
dental implants it has become the mainstream
practice and is clinically accepted as the desired
treatment modality for the patients. Implant
biomaterials, especially bioceramics have provided
the research and dental clinical professionals with
a new essence of interest for over past two decades.
Biomaterial by definition is a “non-drug substance suitable for inclusion in systems which augment or
replace the function of bodily tissues or organs.”
Bioimplants are prosthesis made for regularising
the physiological function of body and bioceramics
represent one classification of the bio implant
based on the material used.
Plaster of Paris (CaSO4.H2O) – first widely evaluated
bioceramic
1892—Dressman published first report on the use
of plaster of Paris to repair bone defect.
1920—first successful use of Tricalcium phosphate
1930—polymeric implants introduced (Rock 1933-
alumina)
1960s—1970s – interest in bioceramic invention
by work of Hulbert and co-worker.
1969-1971—bioactive glass ceramic first introduced
by L.L Hench.
1988—plasma sprayed hydroxyapatite first used
by Herman
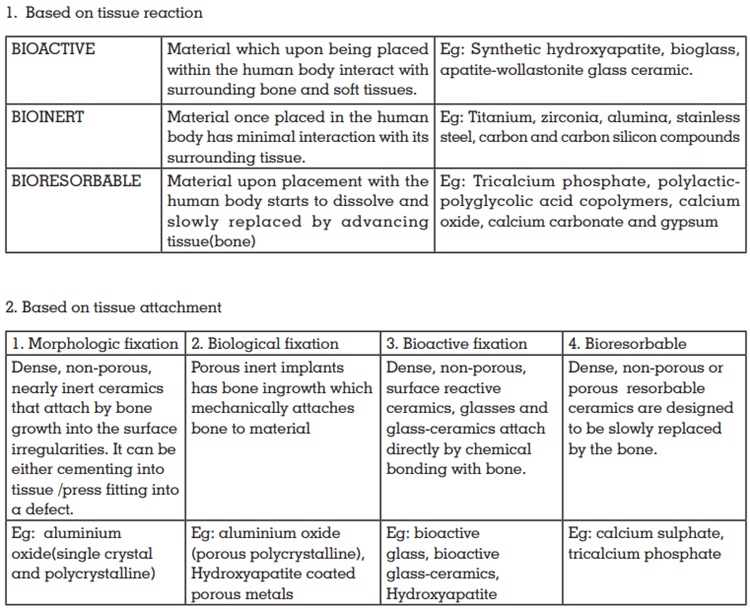
The American dental association outlines some acceptance guidelines for dental implant biomaterials:
Ceramics used for the repair and reconstruction of
diseased or damaged parts of the musculoskeletal
system termed as bioceramics, maybe categorized
as follows:
Bioglass / Glass Ceramics:
Discovery of Bioglass was by Hench and Wilson. It
was first introduced in the year 1971. The glasses
containing specific proportions of silica, sodium
oxide, calcium oxide and phosphorus pentoxide
are termed bioactive. The nucleation and growth
of crystals within the glass converts the glass to
glass ceramics, which retain the bioactivity. They
have high mechanical strength, fast setting ability,
low resistance to tensile and bending stresses and
extreme brittleness. They chemically bond to bone
due to formation of calcium phosphate surface
layer. Ceravital silica is a type of glass ceramic.
Hydroxyapaptite:
It is chemically calcium phosphate [Ca10 (PO4)6(OH)2] and is similar to the mineral component
of bones and hard tissues. Hydroxyapatite was
successfully used as an implant material in 1988
soon after the bioactive glasses were developed.
The hydroxyapatite in powder form is excellent
bone filler. They have calcium to phosphorus
ratio of 1.67 and is the most stable phase of
various calcium phosphates. The preparations
of hydroxyapatite powders include wet methods
and solid state reactions. Hydroxyapatite is stable
in body fluid and in dry or moist air upto 1200°C
and does not decompose.
Plasma sprayed hydoxyapatite was first used
by HERMAN in 1988. It is used as coatings on
implants.
Alumina:
Alumina is a highly inert material which was
introduced by Rock in 1933. It was first used as an
implant material in the 1970s. It has excellent wear
resistance and surface hardness. Alumina exists
in many forms and these arise during the heat
treatment of aluminium hydroxide or aluminium
oxy hydroxide. However the body recognises it as a foreign material and attempts to isolate it
by forming a non adherent fibrous layer which
is considered as a drawback in the use of this
material.
Titanium:
Titanium was first introduced in the year 1789
by Wilhelm Gregor. Due its excellent property
of biocompatibility and its ability to form stable
oxides it has been successfully used as an implant
material in the recent years. Three different oxides
formed on titanium surface are TiO (Anastase),
TiO2 (Rutile) and Ti2O3 (Brookite).
Titanium oxide layer exhibits low level of charge
transfer. Its modulus of elasticity of is half of the
other alloys 5 to 5.6 times greater than bone that
helps in its uniform stress distribution.
Zirconia:
Zirconium dioxide was first extracted from the
mineral Zircon (Zirconium Silicate ZrSiO4) by
the German chemist Martin Heinrich Klaproth
(1743-1817). It was in 1969 the first scientific study
of outstanding biomedical properties of zirconia
emerged and subsequently it was found that
alloying zirconia with oxides of yttria, calcia and
magnesia made it stable. This discovery also led to
the use of the so-called transformation toughening
of zirconia to produce ceramics with unsurpassed
crack resistance (‘ceramic steel’).
Zirconia was successfully used as implant material
in 1960s. It has high flexural strength, fracture
toughness and ability to be polished to a superior
surface than alumina. Zirconia implants also
absorb water and hence become prone to fracture.
Yttrium stabilised tetragonal polycrystalline
zirconia:
This form of zirconia offers best mechanical
properties.
Carbon and carbon silicon
compounds:
Vitreous carbon and carbon compounds are used
in implantology since 1970. Carbon is a versatile that exists in many forms. The biocompatibility
of carbonaceous material to bone indicates its
use in orthopaedic implants. However due to the
intrinsic brittleness and low tensile strength, carbon
compounds have limitations for use in major load
bearing applications.
Calcium phosphate ceramics:
Calcium phosphate ceramics was first commercially
used as implant material in 1980s. They have
biochemical composition similar to bone and
exhibit direct chemical bonding to surrounding
bone. Therefore they are used as implant material
to be gradually substituted by newly formed bone
and get integrated with the host bone. The first
stage is interaction with collagen in bone and
then accumulation of protein and cells on the
surface of the biomaterial and this is followed by
the resorption of the material and finally bone
formation.
A subclass of these ceramics is tricalcium
phosphate ceramics. They are extensively used
owing to its biocompatibility characteristics.
They also have an added advantage of being
resorbable.
Other calcium phosphate compounds include:
Titanium-Zirconium alloy (Straumann
ROXOLID)
Narrow diameter implants (Roxolid®, Straumann, Basel, Switzerland) has recently been introduced
in dentistry. This alloy which has a metallic gray
appearance contains 83-87% titanium and
13-17% zirconium. It has superior mechanical
characteristics over commercially pure (CpTi) and
Ti-6Al-4V, as well as increased fatigue strength.
The addition of zirconia to titanium leads to
excellent osseointegration capabilities. The
biocompatibility of titanium-zirconium alloy is
also more when compared to pure titanium.
In order to maintain the clean oxide layer with
its hydrophilic properties the Titanium-Zirconium
implants are manufactured with the SLActive
surface like the titanium SLActive implants: Sand
blasted, acid etched and then stored in 0.9% NaCl
solution.
Polyetheretherketone (PEEK)
For patients with high aesthetic requirements,
the new material known as PEEK (polyether ether
ketone) is recommended as it is aesthetic, stable,
biocompatible, lighter degree of discoloration.
BioHPP (High Performance Polymer) is based
on polyether-ether-ketone (PEEK) polymer and
was introduced as dental material for precise
prosthetic restoration fabrication. BioHPP has a
low specific weight that permits the fabrication
of lighter prostheses which provides high patient
satisfaction and comfort during masticatory
function.
BioHPP reduces the stress caused by natural
forces as well as the forces attributed to the
prosthetic restorations. While comparing with
titanium, zirconium or ceramic, rehabilitation using
BioHPP significantly reduces the peak masticatory
forces both for axial and oblique movements. This
property provides a positive effect for the patient
and also it extends the durability of the restoration.
Silicon nitride (Si3N4) ceramics
Titanium has been the choice for dental implant fabrication for many years owing to its superior
mechanical and biological performances. The
increasing demand for metal-free restorations has
led to development of ceramic-based implants
like Zirconia and other alternative biomaterials
like PEEK and silicon nitride
Silicon nitride has following properties:
Silicon nitride derives its strength and toughness
through its microstructure, which is mainly
composed of asymmetric needle-like interlocking
grains surrounded by a thin (<2mm) refractory
grain-boundary glass. Unlike other ceramics, there
is no phase transformation is involved.
The primary requisite for a dental implant
material is to be biocompatible and have
superior biomechanical properties. Various
implant biomaterials like titanium, zirconia, etc
are used in this aspect which provides excellent
osseointegration with the bone. The recent
developments in biomaterials having high esthetic
performance like polyetheretherketone and silicon nitride have given way for more future research
which could be of great interest for oral use.