Aim: To evaluate the accuracy of Type I implant
placement using static and dynamic guides compared
to the conventional freehand method.
Settings and Design: This systematic review and
meta-analysis followed the Preferred Reporting Items
for Systematic Reviews and Meta-Analyses (PRISMA)
guidelines.
Methods and Materials: An electronic search of
PubMed (including MEDLINE), EBSCOhost databases,
Cochrane Library, and Google Scholar search engine
for articles published from 1st January 2013 to 1st
March 2023 was conducted. The literature search
intended to retrieve all relevant clinical and in
vitro studies about the accuracy of type I implant
placement using static and dynamic guided surgery
and conventional freehand implant placement.
Statistical analysis used: Meta-analysis was
conducted from the reported quantitative data.
Results: A total of 1486 articles were obtained via
electronic search, and 2 articles were obtained via
manual search; 6 studies met the inclusion criteria
and were included in this systematic review. Among the different parameters described, the difference
in accuracy between virtual and planned implant
positions was evaluated. Accuracy was measured
by evaluating the pre- and post-operative CBCT.
Three studies comparing the accuracy of static guides
with freehand implant placement and two studies
comparing the accuracy of static guides with dynamic
guided implant placement were included for meta
analysis.
The comparison between static and freehand
placement showed a statistically significant difference
between placement accuracy, favouring static
placement, and the comparison between static and
dynamic placement favoured dynamic placement.
Conclusion: The accuracy of type I implant placement
was enhanced using both static and dynamic guided
surgery as compared to the conventional freehand
protocol. Dynamic guided surgery showed greater
accuracy as compared to the static guided system
for type I implant placement.
Key words: accuracy, dental implants, computer-assisted surgery, static guides, freehand surgery
The use of dental implants has become an
integral treatment modality in dentistry for the
treatment of complete and partial edentulism.
Dental implants have several advantages over
conventional fixed partial dentures, like a high
success rate, improved maintenance of bone,
conservation of tooth structure, and decreased
sensitivity of adjacent teeth.1 However, proper
positioning and angulation of implants are
essential for the success of the surgical and
prosthetic treatment.2
There are four basic types of implant placement
depending on the time required for healing
after implantation – Type I: Immediate implant
placement which is done at the time of extraction;
Type II: Early implant placement which is done
4-8 weeks after implant placement with soft tissue
healing; Type III: Early implant placement which
is done 12-16 weeks after implant placement
with partial bone healing and Type IV: Delayed
implant placement which is done after 6 months
of implant placement with complete bone
healing.3 The conventional placement method
(type IV), which requires 3-6 months for healing
before implantation, is the most commonly used.
However, in recent times, immediate implant
placement (type I) is increasing in demand for
its obvious advantages: shortened treatment
time, less surgical trauma, and excellent
treatment outcomes.1 However, several studies
have demonstrated that type I surgery is highly
technically sensitive. Improper position of
immediate implants may lead to restoration and
aesthetic problems, and even peri-implantitis.2
In conventional protocols, implants are placed
freehand, but they are unable to reproduce
the planned implant position accurately. At
present, immediate implant placement depends
on preoperative X-ray or cone-beam computed
tomography (CBCT) assessment, including bone height at the implant area and position of the
lower alveolar nerve and maxillary sinus.4
The aim of surgical guides is to reduce the
inherent positional uncertainty associated with
freehand surgery. Such guides are classified
into two main categories: static and dynamic.
The accuracy of template-based static systems
is acceptable in most clinical situations. Such
templates are mostly fabricated via 3D printing
based on digital images (CBCT/intraoral
scanner), and the resulting template is either
bone-supported, mucosa, or tooth-supported. The
success of implant insertion using static systems
is based on the surgical guide and the doctor’s
experience. A study conducted by Shah et al. in
the year 2022 demonstrated that in the process
of manufacturing static surgical guides, errors
can be introduced, which can result in errors in
the final implant position.5 In comparison with
the original design, the traditional method can
result in an angle or depth that is inconsistent.
This is due to a deviation in the thickness of the
surgical guide and variation in the surgeon’s
experience.6
Prosthetic-driven implant placement for optimal
esthetic restoration has been increasing in
demand during the last decades but requires
higher accuracy. In recent years, machine
vision (MV) enhanced and artificial intelligence
assisted dynamic navigation (DN), a technology
that already has a large number of applications
in industry, is gradually being applied to
image-guided and minimally invasive surgical
approaches and holds great promise for safety
and accuracy.7
Computer-assisted implant surgery (CAIS),
which includes static and dynamic systems,
offers reliable results.6 However, few studies
have evaluated this accuracy for type I implant
placement, which is in demand due to its shortened clinical procedure.
Therefore, this study evaluates the accuracy of
static and dynamic guides for type I implant
placement. The purpose of this study is to evaluate
the accuracy of type I implant placement using
static and dynamic guides as compared to the
conventional method.
This systematic review was conducted according
to the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA)
guidelines8 with prior registration in PROSPERO
(Registration number CRD42023431317).
The focused question was “Is the accuracy of
type I implant placement enhanced using static
and dynamic guided surgery as compared to
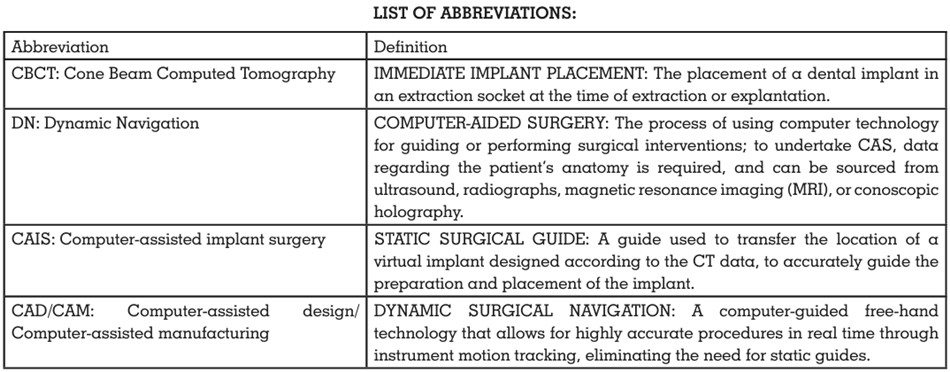
the conventional method?” The PICO, i.e., the Population, Intervention, Comparison, and
Outcome format, was used (Table 1).
The inclusion criteria were in vivo and in vitro
studies that evaluated the accuracy of type 1
implant placement using computer-guided,
static-guided guided and freehand surgery,
studies published in peer-reviewed journals,
articles appearing in the English dental literature,
published between 2013 and 1st February 2023.
The exclusion criteria were studies that were not
related to type I implant placement and studies
in medically compromised patients. Review
articles, case series, and case reports were also
excluded.
An electronic search of PubMed (including
MEDLINE), Cochrane Central, EBSCOhost
databases, and Google Scholar search engine
for articles published from 1st January 2013 to
1st March 2023 was conducted. The controlled
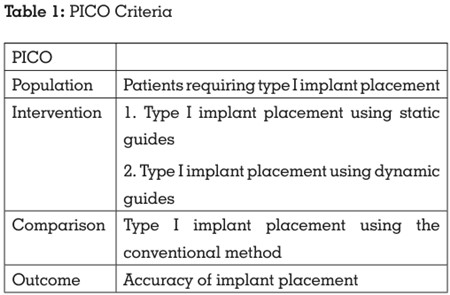
vocabulary terms (i.e., MeSH terms) and free text
terms were obtained by searching key concepts
in the MeSH database and a thorough evaluation
of related articles, thesaurus, dictionaries, and
entry terms. The terms such as dental implant,
dental implants, type I implant placement,
immediate implantation, static guides, surgical
guides, computer-assisted surgery, surgical
navigation, freehand type I implant placement,
conventional type I implant placement,
dimensional measurement accuracy, immediate
implant placement accuracy, type I placement
accuracy were combined using suitable Boolean
operators (AND, OR, NOT) (Table 2).
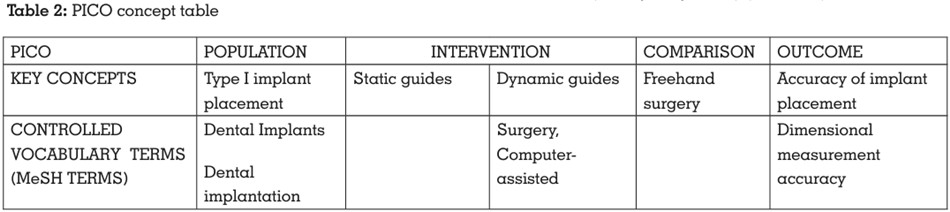
An electronic search was conducted
independently by two reviewers (P.H., R.M.). A
total of 1488 articles were obtained via electronic
search. The articles thus obtained were
evaluated for duplicates. A detailed summary of
data selection has been put forth in the PRISMA
2009 Flow Diagram8 (Figure 1).
The study characteristics of each systematic
review were extracted, including study details,
search details, analysis, and results/findings by
two independent reviewers (P.H., R.M)
A third reviewer (N.P.S.) was called in for a final
decision if any disagreement persisted between
the two calibrated reviewers.
The 1488 articles that were obtained through the
electronic searches were compared meticulously
concerning the author’s name, year of
publication, title, abstract, as well as the journal
name, issue, and volume number. The articles
thus obtained after the electronic and manual
searches were evaluated for duplicates using
the Mendeley Desktop software (v1.19.6). The
2 articles obtained through the manual search
were added manually using the ‘add entry
manually’ feature of Mendeley Desktop software
(v1.19.6). The ‘check for duplicates’ feature of this
software was then used to identify and eliminate
duplicates. 956 duplicate articles were identified
and subsequently eliminated, leaving behind
530 articles. Two calibrated reviewers (P.H., R.M.)
independently screened the relevant titles of the
studies found through the electronic search. Out
of 530 articles, 37 articles were excluded after
screening of the title. The articles thus eliminated
were either literature reviews, scoping reviews,
case reports, case series, or articles not related to type I implant placement. Thus, 493 articles
were selected after title screening.
Two calibrated reviewers (P.H., R.M.) now
independently screened the abstracts of the
studies found relevant during the screening of
the titles, and a total of 478 articles were further
excluded after abstract screening. The articles
eliminated through abstract screening mainly
involved different types of implant placement
and had no comparison groups. 15 articles
were included after abstract screening. Hence,
6 articles were selected after abstract screening
and thus were included in this systematic review.
The 6 articles included 2 in vitro studies, 3
randomised controlled trials, and 1 prospective
study (Table 3).
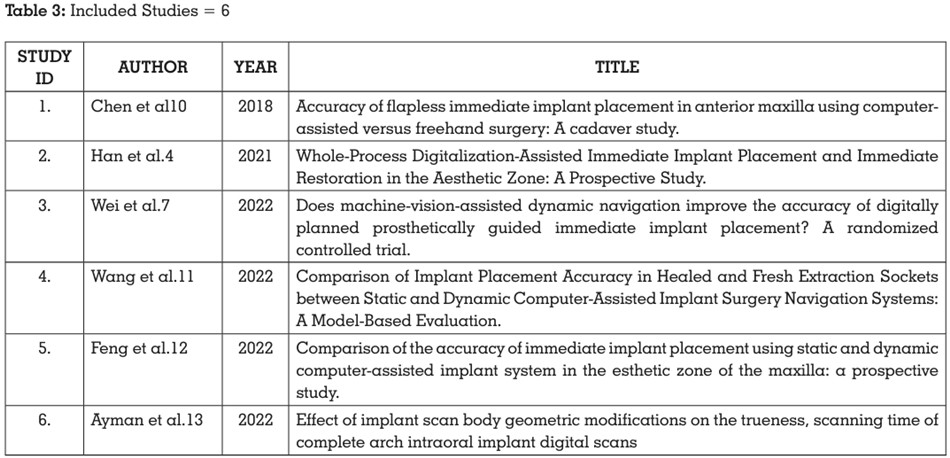
3 articles were excluded after full-text screening.
The reason for exclusion is depicted in Table 4.
A third reviewer (N.P.S.) was called in for a
final decision if any disagreement over article
selection persisted between the two calibrated
reviewers. Inter-reviewer reliability was checked via Cohen’s kappa coefficient.9 The Cohen’s
kappa coefficient values obtained for title,
abstract, and full text screening were 0.62, 0.68,
and 0.75, respectively, indicating moderate inter
reviewer agreement for title, abstract, and full
text screening.
The data were subsequently extracted from the
6 included studies and recorded in 2 Excel data
extraction sheets as mentioned in the summary
table (Table 5).
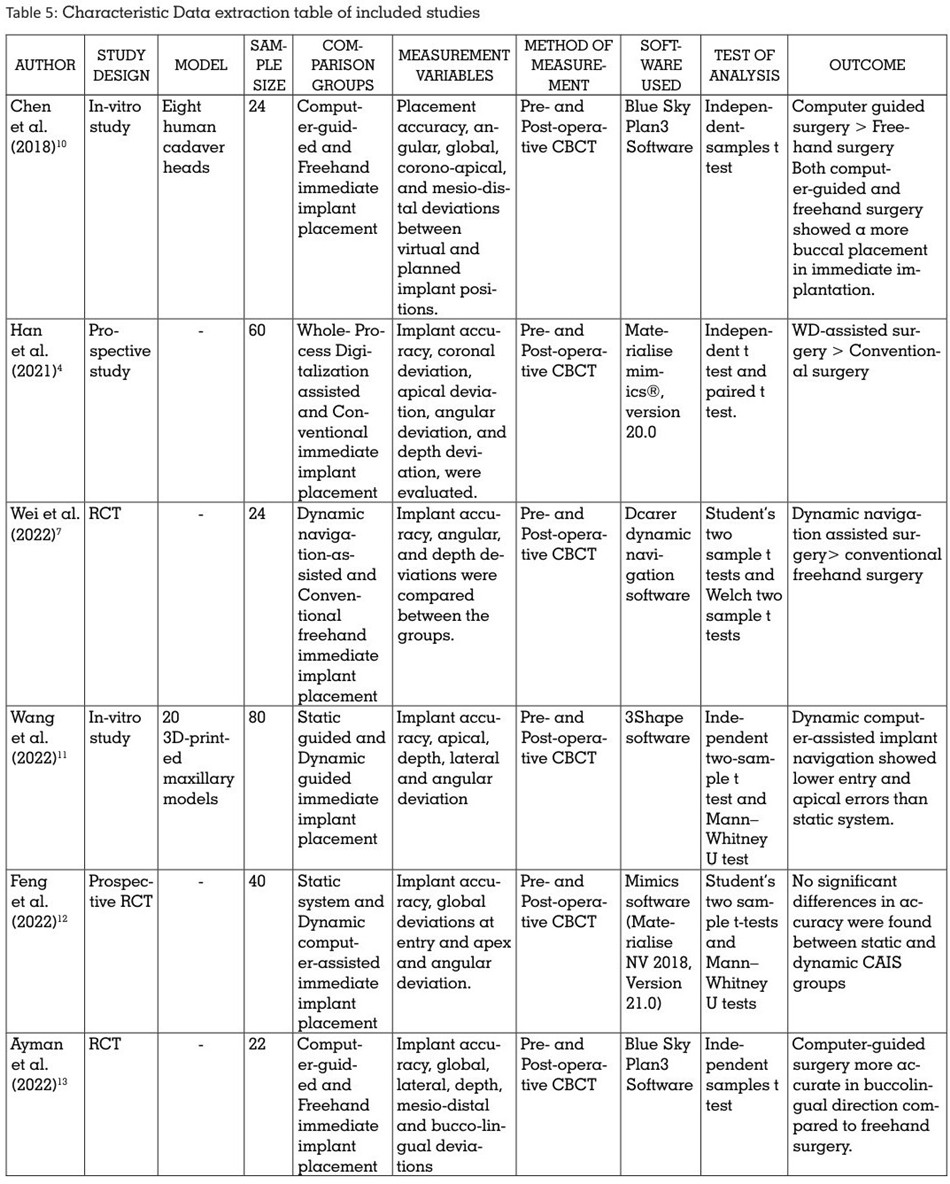
The data extracted was entered under the
following headings: Author and Year of
publication, Study design, Study model,
Sample size, Comparison groups, Measurement
variables, Method of measurement, Software
used, Test of Analysis, and Outcome.
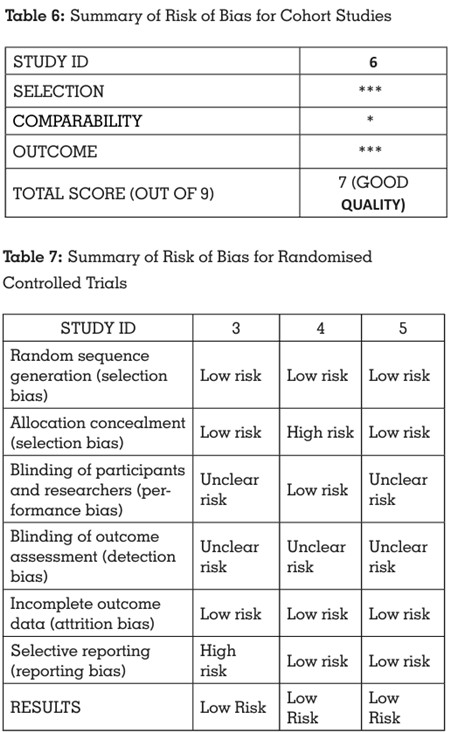
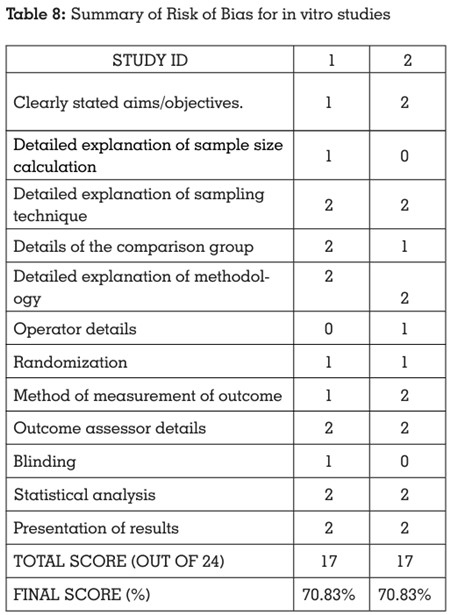
Risk of bias assessment of the included studies
was done using the Newcastle-Ottawa scale15 for
cohort studies, Cochrane’s Collaboration tool16
for randomised controlled trials, and QUIN tool
scale17 for in vitro studies, by two independent
reviewers (P.H., R.M.).
These scales were considered apt for the risk of
bias evaluation in this systematic review. The
changes made to the scale were validated by
the third reviewer (N.P.S.).
All the included studies showed low risk of bias, indicating a high quality of evidence. The scores
were categorized as:
Low risk of bias (plausible bias unlikely to
seriously alter the results); Unclear risk of bias
(plausible bias that raises some doubt about the
results); or
High risk of bias (plausible bias that seriously
weakens confidence in the results) for the in vitro
studies and randomized controlled trials; and
Good quality (plausible bias unlikely to seriously
alter the results), Fair quality (plausible bias that
raises some doubt about the results), or Poor
quality (plausible bias that seriously weakens
confidence in the results) for the prospective
study.
Summarized results indicate an overall high
quality of the included studies, with a high risk
of bias being present only for specific points
(Tables 6, 7, 8).
META ANALYSIS
Six studies evaluating the accuracy of type1
implant placement with the use of static
and dynamic guides in comparison with the
conventional implant placement were included
in the systematic review.
One study, which compared the accuracy of dynamic navigation-assisted immediate
implant placement with the conventional
freehand technique (Shi-Min Wei et al., 2022)7
was excluded from the meta-analysis due to
a lack of uniformity in the comparison groups.
Three studies comparing the accuracy of static
guides with freehand implant placement (Doaa
M Ayman et al., 2022; Xiaomei Han et al., 2021;
Zhaozhao Chen et al., 2018)13,4,10 and two studies
comparing the accuracy of static guides with
dynamic guided implant placement (Miaozhen
Wang et al., 2022; Yuzhang Feng et al., 2022)11,12
were included for meta-analysis.
The Review Manager software (Version 5.4.1)
was used to perform meta-analysis. Mean values
and standard deviations for coronal and apical
deviation were included for the analysis.
The data was tabulated under the headings of
study name, group, and effect size. The effect
size was calculated on the continuous raw
data entered for mean, standard deviation,
and sample size. A 95% confidence interval
for each effect size was also computed. The
heterogeneity of effects was assessed by the
Higgin’s I2 test.18 The I2 statistic describes the
percentage of variation across studies that is
due to heterogeneity rather than chance and
is denoted by the formula: I2= 100% x (Q-
df)/Q. According to Higgins et al, calculation
of heterogeneity is essential in determining the
generalizability of the findings of meta-analysis.
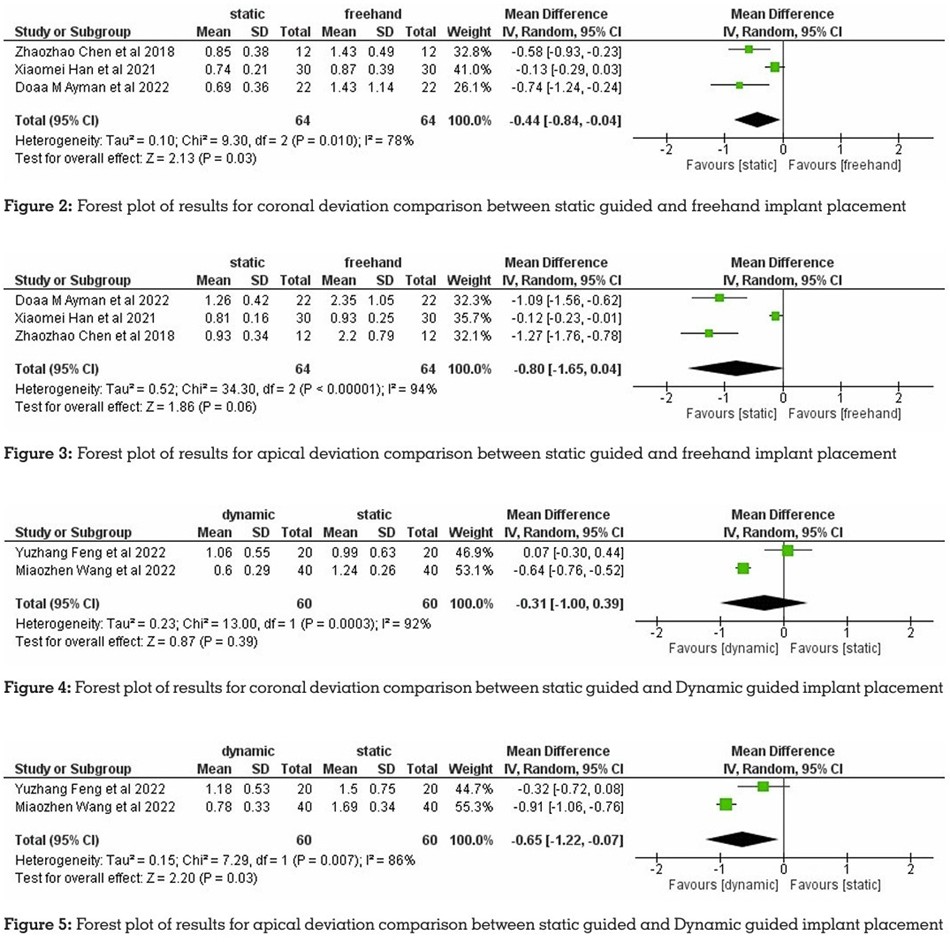
The results of the meta-analysis comparing
the coronal deviation between static guided and
freehand implant placement showed a greater
accuracy of implant placement with static guided
surgery. The meta-analysis comparing apical
deviation between static guided and freehand implant placement also showed greater accuracy
with static guided surgery. The results of the
meta-analysis comparing the coronal deviation
between static guided and dynamic guided
implant placement showed lesser deviation with
dynamic guided surgery. The meta-analysis comparing apical deviation between static
guided and dynamic guided implant placement
also showed greater accuracy with dynamic
guided surgery (Figures 2, 3, 4, 5, respectively).
The development of immediate implant
placement has provided a solution to problems
caused by delayed implant treatment. However,
accurate transferring of the preoperative
implant plan to the surgical site is essential for
appropriate restoration to ensure functional and
aesthetic outcomes.19
The conventional freehand immediate implant
placement can lead to irregular extraction socket
shape, poor restoration shape, mechanical
complications, poor self-cleaning ability, and
other issues.20
To solve such problems, digital-assisted
immediate implant placement has attracted a
large amount of attention. The advent of this
technology has paved the way for a highly precise
and efficient digital workflow.13 CAD technology
can accurately reconstruct 3D models of patients
and enables dentists to design implants in 2 or
3 dimensions.20
Studies have reported that digital technology
helped to determine the optimal 3D position of
the implant in the software and helped control
implant position precisely.14 By constructing
the 3D whole-process guide plate with CAM
technology, the implant can be accurately
implemented in surgery. Compared with
conventional implantation, digital-assisted
implantation was not only more accurate but
also preserved the peri-implant bone tissue.20
Computer-assisted implant placement
nowadays typically contains static and dynamic
technological pathways. A significantly higher accuracy of implant placement was achieved
with both systems as compared to the freehand
protocol, as suggested by clinical evidence.6
The studies evaluated in this systematic review
compare the accuracy of immediate implant
placement with static and dynamic guides
and the freehand placement protocol. The
difference between the planned and the actual
implant positions was measured. The method
of measurement used in all the studies was
pre- and post-operative CBCT images. The
measurements were made for global, coronal,
apical, and angular deviations. Among the
parameters described were implant placement
accuracy, global deviations at entry and apex,
angular deviations between planned and
postoperative implant positions, and deviation
of implant placement at mesial-distal, labial
palatal, and coronal-apical directions.
There were three studies (Ayman et al., 2022; Chen
et al., 2018; Han et al., 2021)13,10,4 comparing the
accuracy between static guided and freehand
implant placement, two studies (Wang et al., 2022;
Feng et al., 2022)11,12 comparing static guided
and dynamic guided implant placement and
one study (Wei et al.,2022)7 comparing dynamic
guided and freehand implant placement.
The studies evaluating the accuracy between
static guided and freehand groups showed a
greater accuracy of implant placement with the
static guided placement. The studies comparing
static and dynamic guided groups showed
a greater accuracy with the dynamic guided
implant placement, and the study evaluating
the dynamic guided and freehand implant
placement showed a more accurate implant
placement with dynamic guided implant
placement.
The risk of bias analysis was done by the
Newcastle-Ottawa scale15 for cohort studies, the Cochrane Collaboration’s Tool16 for randomized
controlled trials, and the QUIN Tool17 for in vitro
studies.
Five included studies were homogenous in their
study design and outcome variables. Hence, a
quantitative analysis through a meta-analysis
was planned. The results of the quantitative
analysis have been provided in the form of forest
plots for easy visualization.
The heterogeneity of the primary studies has
been evaluated using the Higgins’ I2 test.18
Heterogeneity refers to differences in results
between primary studies that are greater than
expected by chance alone.
The results of the meta-analysis for the three
studies comparing coronal and apical deviation
between static guided and freehand implant
placement showed a greater accuracy with
static guided surgery. The coronal and apical
deviation comparison between static guided
and dynamic guided implant placement, which
was evaluated in two studies, showed greater
accuracy with dynamic guided surgery.
Thus, this systematic review reports an overall
better implant placement accuracy with static
and dynamic guided immediate implantation
as compared to the conventional freehand
immediate implant placement.
Limitations of this systematic review were; The
search for this study was limited to articles
published in the English language, grey literature
hasn’t been searched for relevant literature
which could have resulted in mild selection bias
and, the results of this systematic review should
be applied with caution to the clinical scenario
since all the included studies are not clinical
studies with some included in-vitro studies.
The implant placement accuracy is significantly
dependent on the method of implantation.
Within the limitations of this systematic review,
the following conclusions could be drawn:
1. The accuracy of type I implant placement
was enhanced using both static and dynamic
guided surgery as compared to the conventional
freehand protocol.
2. Dynamic guided surgery showed greater
accuracy as compared to the static guided
system for type I implant placement.
However, more clinical studies are necessary for
safer conclusions, since the available scientific
evidence is not yet conclusive.