

Composite defects of the head and neck region after oncologic resection are challenging and require reconstruction of several layers, including the intraoral lining, osseous reconstruction of the mandible or maxilla and soft tissue/skin coverage. Management of complications resulting from flap failure is a challenging task from a technical and aesthetic perspective that can have a substantial social and psychological impact on those affected. This clinical report describes prosthetic rehabilitation of a lateral midfacial defect and the clinical challenges encountered and their solutions in a patient with carcinoma of gingivobuccal complex who underwent composite bite resection, reconstruction and adjuvant radiation therapy. A conventional approach that employed an acrylic substructure and silicone (one-piece) prosthesis was implemented to address the cutaneous cheek defect taking into account history of recent radiotherapy and comprehensive medical history. The delivered prosthesis effectively restored the lost facial contour and concealed the facial defect, contributing to aesthetics and improving the patient’s quality of life.
Key words: facial prosthesis, extraoral prosthesis, maxillofacial, silicone, squamous cell carcinoma.
The gingivobuccal complex (GBC), includes the
buccal mucosa, upper and lower gingivobuccal
sulci, alveolus and retromolar trigone, is a
common subsite for oral cancer.1
Squamous
cell carcinoma (SCC) of the GBC is uncommon in Western countries, accounting for only 10%
of oral cancers. However, it accounts for 40% of
oral cancers in Southeast Asia, South-central
China and Africa.2
This can be attributed to the
widespread use of smokeless tobacco in the form
of chewing tobacco, nut, and lime. Compared
with other common oral cancers, such as tongue
and floor of mouth cancer, SCC of GBC readily
infiltrates the buccinator muscle and buccal pad
of fat, more easily invades the mandible and
skin and spreads to cervical lymphatic tissue.2,3
Reconstructing composite defects in the head
and neck region after oncologic resection
involves the reconstruction of multiple layers
such as intraoral lining, osseous reconstruction
of mandible or maxilla, and soft tissue/skin
coverage to achieve adequate sealing of
the intraoral defect and visually appealing
external skin coverage capable of withstanding
adjuvant radiation therapy.4-6 The pectoralis
major myocutaneous flap (PMMC) remains
the flap of choice for reconstruction of complex
full-thickness defects in the head and neck
region following ablative resections, despite the
availability of microvascular surgery and other
free flap reconstructions.7,8 Noteworthy benefits
of PMMC flaps include good vascularity, short
learning curve, and reduced requirement for
specialized equipment.6
Potential complications
include orocutaneous fistula (5.2%), partial flap
loss (3.5%), flap dehiscence (1.7%), hematoma
(1.7%), donor site abscess (1.7%), plate exposure
(1.7%).1,10
Vartanian et al. have reported low complication
rates with the PMMC flap, for complete
and partial flap necrosis of 2.4% and 9.7%,
respectively, in 371 cases.11 The most common
complication is dehiscence of the sutures, which
can lead to salivary leakage and secondary
infection. It can lead to prolonged hospital stay,
delay recovery and significantly increasing
morbidity.12 Management of a defect resulting from flap failure is a challenging task from a
technical and aesthetic perspective.9,13 Many
times secondary reconstruction of the defect
is not a feasible option, due to the lack of
availability of tissue, the impact of irradiation
on the local vascular bed in tumour patients,
and the patient’s physical condition.14,18,19 It is
not uncommon for surgeons to wait at least a
year after a major resection before considering
surgical reconstruction of a facial defect caused
by a flap complication or the tumour itself.15
Thus, a facial prosthesis (interim or definitive)
constitutes a viable alternative for many
patients to enhance their confidence, facilitate
social integration, and reduce psychological
burden.16,17
This clinical report describes the prosthetic
rehabilitation of a lateral mid-facial defect
and the clinical challenges encountered and
their solutions. A conventional approach that
employed an acrylic substructure and silicone
(one-piece) prosthesis was implemented, taking
into account history of recent radiotherapy and
comprehensive medical history. The primary goal
was to effectively restore the lost facial contour
and conceal the facial defect, contributing to
aesthetics and improving the patient’s quality of
life.
A 35-year-old young gentleman reported to
our tertiary cancer care centre with a clinical
presentation of ulcero-proliferative growth in the
right buccal mucosa. A computed tomography
of head and neck region suggested well-defined
heterogeneously enhancing lesion involving
both upper and lower GBS, retromolar trigone,
abutting right masseter and medial pterygoid
muscle, erosion of posterior wall of maxilla on
the right side. The patient underwent right bite
composite resection with right neck dissection
and bipaddle pectoralis major myocutaneous flap reconstruction (pT2N0M0). During the initial
postoperative period, the patient developed
seropurulent discharge, parotid leak, and suture
dehiscence with no fever. Following which the
sutures over the outer pedicle were removed
to facilitate pus drainage and betadine wash
followed by application of regular dressing.
After 3 weeks of healing period, the patient was
advised postoperative adjuvant radiotherapy of
60 grays and 30 fractions. During the course of
radiotherapy (19 fractions), flap dehiscence was
encountered involving only the outer aspect of
the flap and no intraoral gaping or discharge.
The patient received a total of 56 grays / 28
fractions and periodic follow-up to assess the
flap site.
Seven months after radiation therapy, the
patient was referred to the Dental and Prosthetic
Surgery Department for an assessment of the
midfacial defect. Clinical examination revealed
a cutaneous defect measuring 3x3 cm, below
the zygomatic arch along the upper border of
the PMMC flap and altered facial contour on the
right side of the face. There was no intraoral or
nasal communication of the defect. (Figure 1)
The mucosal quality on the remaining portion
of the defect showed no signs of inflammation,
residual skin tags, or scar tissue. The junction
between the underlying mucosa and the outer
skin was distinct and healthy. Diminished
vascularity, fibrosis, and scarring of the tissues
surrounding the defect increase the probability of complications associated with secondary
reconstruction. To avoid such risks, the surgeons
opted to postpone secondary reconstruction of
the facial defect for at least a year after head
and neck irradiation. Therefore, prosthetic
rehabilitation was planned during this interim
phase with a conventional approach that utilized
an acrylic substructure and silicone (one-piece)
prosthesis.
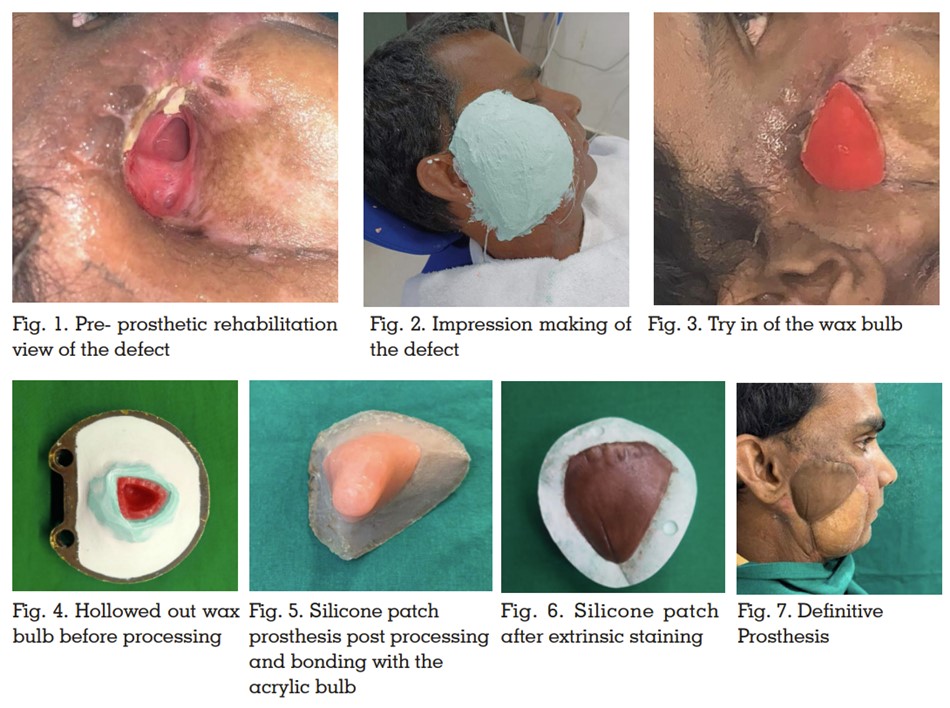
A facial moulage of the defect side was made with
irreversible hydrocolloid impression material to
accurately record the tissue undercuts (Figure2)
and a working model was obtained. On this
model, a wax bulb was fashioned to encompass
the inner aspect of the defect. This was evaluated
on the patient to obtain the appropriate base
for the prosthesis while ensuring passive fit,
no gaping, and undue trauma to the internal
tissue bed (Figure 3). Once satisfactory, it was
hollowed out to ensure it was lightweight and
then processed in acrylic (Figure 4). The acrylic
bulb was assessed on the patient and with
it properly situated within the defect, a pickup impression was made using irreversible
hydrocolloid impression material for subsequent
prosthesis fabrication. A working stone model
was obtained, and tin-foil was adapted. A clay
sculpture was carved to simulate cheek contours
with proper margin placement. A clinical trial was
performed and modified as necessary. Standard
laboratory steps were followed for investing,
dewaxing, and mould preparation without the
acrylic bulb. Room temperature vulcanising
silicone (A 20001, Factor II Inc., USA) was packed
into the mold space after shade matching with
patient’s skin and intrinsic staining according
to the manufacturer’s instructions. The silicone
patch was recovered, trimmed and bonded to
the acrylic bulb using medical adhesive (Factor
II, Inc) (Figure 5). As the final step, the prosthesis
was clinically evaluated in the patient for
proper fit, intimate adaptation of the margins,
colour and it was extrinsically stained for better characterization and precise matching. Once
cured, it was delivered to the patient (Figure
6 and 7). We did not experience clinically
significant mobility or sinking of the prosthesis
during functional movements due to the light
weight of the prosthesis, use of undercuts and
good support from the remaining orbital roof and
zygoma. Instructions regarding the positioning
and maintenance of the prosthesis were given
and regular follow-up (1 day, 1 week, monthly)
was advised.
The loss of a part of the face can have a
substantial social and psychological impact on
those affected.13 The use of a facial prosthesis
can provide support during the adjustment
process. The facial prosthesis may be made
of silicone, acrylic resin, or a combination of
both. The skin in the cheek area is affected
by facial expressions and jaw movements
and more susceptible to compression due to
the absence of supportive bony structures.14,16
Certain challenges encountered during
clinical procedures for prosthetic rehabilitation
included choice of retention, placement of the
prosthesis margins in natural junction zones of
the face, choice of prosthetic material, accurate
colour matching and static appearance of the
prosthesis.
There are several means of retention used in
maxillofacial prostheses depending on the
size of the defect, the options available, the
patient’s condition, and preference.15 Among the
choices are anatomical undercuts, adhesive,
magnets, implants, and combinations of the
previous means. As for the magnet attachment,
the potential problem of losing its magnetic
attraction must be taken into account.7
Although
craniofacial implants represent a state-of-the-art solution, certain patients may not meet
the criteria for implant intervention due to diverse reasons, such as unfavourable tumour
prognosis, defect location, compromised
irradiated tissue beds, higher susceptibility to
peri-implant skin reactions, and unfavourable
loading conditions.18 Whilst several previous
studies have demonstrated differences in failure
rate of craniofacial implants at irradiated sites, it
is recommended that patients received implants
12 months or more following irradiation.19 The
use of adhesive may act as a potential irritant on
a previously irradiated tissue bed; thus, it was
avoided. Engaging anatomical undercuts in an
atraumatic manner was the choice of retention
in the clinical present case.
Special emphasis was placed on intrinsic
colour matching and margin thickness to obtain
slight pressure on the skin and at the same
time properly adapt to the facial expressions
and jaw movements. The construction of a
silicone patch that adheres to an acrylic resin
substructure effectively addressed the issue of
autonomous mobility within the cheek defect
during mastication and facial movements.
Room temperature vulcanizing silicone
material was chosen as it is easily processed
with readily available instrumentation, has
sufficient flexibility for use on movable tissue
beds that offered a distinct advantage. Shades
in different regions were developed chair side
using extrinsic stains to simulate the lighter and
darker areas present on the patient’s face. In
general, patient acceptance of the prosthesis
was notably improved markedly due to good
retention, favourable aesthetic outcomes
resulting from precise and consistent positioning
of the margins and ease of maintenance.
Reconstruction of oncologic head and neck
defects continues to pose a formidable
challenge, even with recent progress in
surgical reconstruction techniques and history of adjuvant radiotherapy only exacerbate the
difficulties of the reconstruction process. In the
present clinical case, the patient underwent
prosthetic rehabilitation using a one-piece
acrylic-based silicone prosthesis that exhibited
improved functionality, aesthetics, and patient
acceptance.
Authors would like to thank Mr Sagar Kulthe and
Mr Akshay Umale for extending their assistance
in laboratory procedure.