

Peri-implantitis is an inflammatory process which occurs around an osseointegrated implant, resulting in pocket formation and bone loss. Most implant system consist of two pieces; an implant fixture and an abutment, the microgap which exist between them is referred as implant-abutment interface. The aim of this study was to evaluate the adequacy of sealing materials on microleakage at implant-abutment interface.
Key words: Microleakage, Implant-abutment interface, periimplantitis
Peri-implantitis is an inflammatory process which
occurs around an osseointegrated implant, resulting in pocket formation and bone loss1
. Most
implant system consist of two pieces; an implant
fixture and an abutment, the microgap which exist
between them is referred as implant-abutment
interface2
. This gap at implant-abutment interface
offer shelter to the accumulated biofilm which
contain bacteria leading to bacterial colonization
and peri-implantitis3
.
Microleakage has been considered to occur in
both directions from an external source to the
inner area of an implant and vice versa. The gap
between the implant and abutment facilitates the
microleakage4
. During function, bending forces act
on the implant component which losses the screw
joint, thereby increasing the gap. It also produces
the pumping effect to transport the bacteria,
allowing for microleakage5. Various measures have been used to prevent microleakage at implant-abutment interface using sealing material, shape
memory alloy and different connection geometries4
.
The aim of this study was to evaluate the adequacy
of sealing materials on microleakage at implant-abutment interface.
Experimental Groups
In this study, 120 titanium dental implant, standard,
internal hexagon, 3.5mm diameter, and 10mm length were utilized to assess the adequacy of
different sealing materials at IAI (implant-abutment
interface). The samples were divided into three
groups containing forty samples of each group:
Group I: Titanium dental implant with internal
hexagon were connected with straight, titanium
abutment 3mm with a torque of 25Ncm according to
manufacturer instructions, without the application
of sealing material at IAI.
Group II: Titanium dental implant with internal
hexagon were connected with straight, titanium
abutment 3mm with a torque of 25Ncm according to manufacturer instructions with the application
of antimicrobial sealing gel (Gapseal) at IAI.
Group III: Titanium dental implant with internal
hexagon were connected with straight, titanium
abutment 3mm with a torque of 25Ncm according
to manufacturer instructions with the application
of O-ring at IAI.
Preparation of the samples
Under sterile conditions, dental implants and
abutments were removed from commercial
packaging. These samples were cultured for
another 24 hours in a sterile nutrient solution, to
ensure complete sterilization. The sample that
fulfilled the criteria was selected for the study.
Revival of staphylococcus aureus from
freeze-dried culture powder
Freeze-dried culture powder of staphylococcus
aureus (MTCC 3160) was revived by incubating the culture powder in nutrient broth for 24 hours
under a sterile environment. 50ml of this suspension
were transferred on Tryptic Soy Agar plate using a
sterile loop. The bacteria were streaked across the
plate from left to right and top to bottom and the
plates were incubated for 12 to 16 hours at 37°C to
obtain isolated colonies of staphylococcus aureus.
Preparation of inoculum
The cultures of Staphylococcus aureus (MTCC
3160) onto Tryptic Soy agar were used to prepare
a bacterial suspension of about 1 x 108 colony
forming units (CFU/ml) in nutrient broth by
adjusting turbidity to 0.5
Experimental procedure
The experimental procedure was carried out
under aseptic conditions. The working area was
disinfected with 70% ethanol before starting the
procedure. The aseptic conditions were maintained
by following routine measures such as using sterile
gloves, sterile equipment, eye protection, Bunsen
burner, and laminar flow cabinet. The implant
and abutment from each group were attached
and immersed into 3ml of bacterial suspension
inoculated with Staphylococcus aureus that covered
the IAI. These samples were further incubated at
37°C for 24 hours. Later, the assemblies were
removed from the bacterial suspension and the
external surface is decontaminated with a 2%
solution of sodium hypochlorite for 30 minutes.
The residual sodium hypochlorite was removed
with normal saline.
To check the adequacy of the external surface
decontamination strategy, the assemblies were additionally placed in sterile nutrient solution and
incubated for 24 hours at 37°C.
After decontamination, the implant and abutment
assemblies were disassembled and submerged
into sterile nutrient solution in the test tubes.
The test tubes were agitated so that nutrient
solution sufficiently contacts the inner surface of
the implant and abutment assemblies, allowing
the bacteria to flow into the solution. Nutrient
agar plates were divided into four quarters and
were inoculated with 100μl of nutrient solution
(containing staphylococcus aureus). The nutrient
agar plates were then incubated for 24 hours at
37°C.
The resulting colonies were identified and
quantified (FIGURE 1).

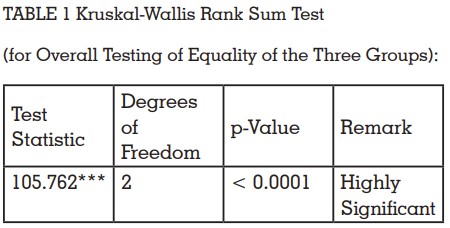
Statistical analysis was performed using
customized R programming software. The data
obtained was subjected to Kruskal Wallis analysis
of variance (Table 1). The level of significance was
set at p ≤ 0.05. Statistically, a significant difference
was found between the three groups (P-value
<0.001). The Mann-Whitney U-test was applied to
evaluate differences between the three groups with
respect to the mean number of bacterial colonies.
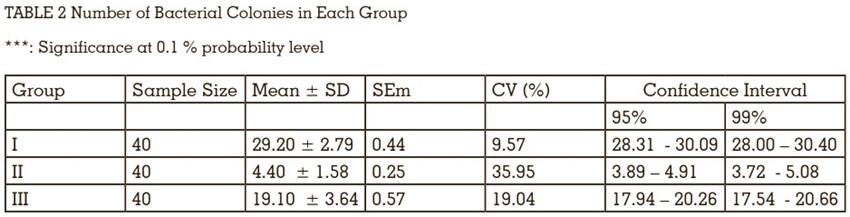
Microleakage occurs in all the groups with or
without sealing material. In group, I maximum
bacterial count was observed, ranged from 25 to
33 (mean, 29.20; standard deviation (SD) +- 2.79).
In group III average bacterial count ranged from
13 to 25 (mean 19.10; SD,+-3.64). However, group
II exhibit the maximum resistance to microleakage,
observing the least bacterial count, ranged from
2 to 6 (mean, 4.40; SD+-1.58) (TABLE 2).
There existed highly significant differences among
the three groups understudy for the average number
of bacterial colonies (FIGURE 2). Consequently, it
becomes imperative to make post-hoc comparisons among their performance, using the Mann-Whitney
U test. Member groups in all the three paired
comparisons showed highly significant differences
(each at 0.1 percent probability level) concerning
the mean number of bacterial colonies (TABLE
3).On average, the number of bacterial colonies
was the minimum (= 4.4) in Group-II, followed
by that (= 19.1) in Group-III and the maximum
(= 29.2) in Group-I.
The present study was conducted to assess the
adequacy of sealing material at the implant-abutment interface to prevent microleakage.
The results showed that bacterial infiltration
of staphylococcus aureus occurs in all three
groups, however, the least amount of bacterial
infiltration was observed with Gapseal followed
by O-ring. Furthermore, the study was conducted
under static conditions, which revealed that the
presence of sealing material help to reduce the
microleakage, but a reliable seal is not obtained
at the interface. The presence of gapseal helps to
reduce the leakage by its antimicrobial properties
or its sealing ability. Gapseal is a highly viscous
silicone material, which allows it efficiently seal
the interstitial spaces, maintaining a complete
seal. It also has hydrophobic properties, which
ensure high retention and prevent it from being
washed away6
. Several studies have shown the
same results7-9.
Paolantonia et al. found that filling the internal
cavity with 1% chlorhexidine gel; significantly
reduce bacterial colonization over a period of
6 months7
. The sealing ability of chlorhexidine
varnish and silicone sealant was tested by Duarte
et al. In vitro, both materials could prevent some
bacterial leakage for a period of 45 to 63 days8
.
Nayak et al. recommended the use of gapseal
to enhance the sealing capability, the viscous
nature of the gel allows it to flow easily throughout
the interfaces2
. Zarbakhsh et al. reported that
gapseal reduces the microgap and prevents the microleakage under cyclic loading9
.
In group III microleakage occurs because the
O-ring prevents the abutment from complete
seating, resulting in increased microleakage at the
implant-abutment interface. Furthermore, rubber
can also deteriorate over time, leading to increase
leakage. Without sealing material microleakage
occurs in group I, which was most likely owing to
the lack of complete wall adaptation between the
implant and abutment assembly2
.
Several investigations have revealed bacterial
leakage along with the implant-abutment interface
of systems with varied connection arrangements10.
Quirynen et al. found that microbe infiltration
occurs into the internal part of the implant which
could be a result of abutment installation or
unscrewing5
. Jansen et al. stated that microleakage
occurs at the implant-abutment interface, even if
the size of microgap was less than 10μm11.
The Rationale to use colonies of Staphylococcus
aureus for the present investigation was the
biological role that, this aerobic bacterium has,
during the initial phase of biofilm development
on the titanium implant surface. It is an initial
colonizer with a strong affinity to attach to other
pathogenic bacteria as well as to any type of
titanium surface12.
Cyclic loading of the implant may also contribute to
microleakage. One limitation of the present in vitro
study is that cyclic loading was not implemented
to mimic masticatory stress. Steinebrunner et al.
investigated bacterial leakage at the implant-abutment interface, following the use of dynamic
loading, which significantly improved in various
implant systems13. According to Nascimento et al.
human saliva can penetrate the implant-abutment
interface under loaded and unloaded conditions14.
Thus, it’s vital to substantiate or contrast the current
study findings with different loading conditions.
Considering the limits of the present in-vitro study
it was concluded that Gapseal was effective in
preventing microbial leakage at implant-abutment
interface followed by O- ring. Further evaluation
is needed about the longevity of the antibacterial
sealing gel.