

Introduction: Glass ionomer luting cements (GIC)
and Resin modified glass ionomer luting cements
(RMGIC) are used to attach and seal fixed dental
prostheses to teeth. Despite of their anticariogenic
properties, there is still existence of caries. Studies
have shown incorporation of chlorhexidine (CHX)
can increase its antimicrobial action without
affecting their physical properties.
Objectives of the study: The objective was to
evaluate the effect of incorporation of CHX on
flexural and compressive strength of conventional
GIC and RMGIC. To compare the strength of both
the cements on incorporation of CHX.
Methodology: Forty bar shaped specimens and
cylindrical specimens of both the cements were
prepared for flexural strength and compressive
strength testing using stainless steel mold. CHX
powder was incorporated into experimental groups
of both luting cements in a concentration of 1%.
Specimens were stored in artificial saliva for 24
hours. Flexural strength and compressive strength
of the specimens was determined using universal
testing machine. Morphological evaluations for
fractured surfaces were done using scanning
electron microscopy. The data was statistically
analyzed using independent sample t-test.
Result: The results of the study showed that, addition
of 1% CHX decreased compressive and flexural
strength of both conventional GIC and RMGIC. On
addition of CHX RMGIC showed better compressive
and flexural strength compared to conventional GIC.
Conclusions: The Chlorhexidine (CHX) amount
should be kept below 1% for both the cements to
sustain their strength.
Key words: Glass Ionomer Luting Cement; Resin Modified Glass Ionomer Luting Cement; Chlorhexidine; Compressive Strength; Flexural Strength
Dental luting cements link fixed dental restorations
and tooth structure. This attachment may be
mechanical, chemical, or a combination of both
methods. In addition to providing a gap-free
interface, the luting cements should ideally also
help to prevent micro leakage and secondary
caries and decrease the failure rate of partial
fixed dental prostheses.1
The ability of glass
ionomer cement to release fluoride continuously
over an extended period of time, results in an
anticariogenic potential showing a reduction in
caries adjacent to the restoration.3
Recently, there has been considerable interest in
luting materials with adhesive capabilities and
therapeutic potential. Glass ionomer cements
(GIC) are acid–base cements which are used
widely in dentistry which have been used more
recently as bone cements.6
Conventional GICs are
complex materials, constituted by a calcium and
aluminium polyacrylate matrix with glass particles
embedded in it.7
Conventional glass ionomer
luting agents have fluoride ion release, physio-chemical bonding to tooth structure, and a low
coefficient of thermal expansion.8,9 Resin-modified
glass ionomer cements also release fluoride and
contain resin components for improved physical
and mechanical properties.10 Resin luting agents
are required for cementation of porcelain veneers,
all-ceramic crowns, and indirect composite or
ceramic restorations and are now available in
autopolymerization, light-polymerization, and
dual-polymerization formulations. Several attempts
in developing GIC with enhanced antibacterial
effects by addition of bactericides, such as,
chlorhexidine hydrochloride, cetyl pyridinium
chloride, cetrimide, and benzalkonium chloride
have been reported in the literature.11 Two groups
of bacteria are responsible for initiating caries:
Streptococcus mutans (SM) and Lactobacillius
casei (LB).12,13 Among the different antimicrobial
agents used to control dental microorganisms
the use of chlorhexidine (CHX) mouth rinses to control dental plaque and gingivitis has been
well established.14 Chlorhexidine has been
considered as one of the most effective and
safe substances.15 Therefore, to provide specific
and continuous antibacterial protection against
complex microorganisms residing between the
teeth and fixed restorations, incorporating CHX
may improve clinical success. The addition of small
concentrations (1%) of chlorhexidine increased
the antibacterial activity without compromising
the mechanical properties.18
The luting cements are subjected to compressive
and tensile stresses by masticatory forces.19
Luting cements must withstand masticatory and
parafunctional stresses for many years in a warm
and wet oral environment.20
Two glass ionomer cements were employed in
this study, a conventional glass ionomer luting
cement (Fig. 1) which was grouped as A and a
resin-modified glass ionomer luting cement (Fig
2) which was grouped as B. For each cement
type twenty bar shaped specimens of dimension
25×2×2mm (according to ISO Standard-4049)6
were made to test the flexural strength (grouped as
Fs) and twenty cylindrical specimens of dimension
12×6mm (according to ANSI/ADA Specification no
66)19 were made for compressive strength testing
(grouped as Cs) using a brass mold of appropriate
dimension as shown in Fig 3 and Fig 4 respectively.
The molds were coated with petroleum jelly. Control
group contained twenty specimens for each type
of cement, which were prepared according to
recommended powder liquid ratio for testing
both flexural and compressive strength. Three
measuring spoons of powder and 3 drops of liquid
were necessary to fill the 12mm x 6mm matrix,
three measuring spoons of powder and 3 drops
of liquid were necessary to fill the 25×2×2mm
matrix. They were manipulated over a mixing
sheet using plastic spatula which were supplied
by the manufacturer. A plastic plate was placed below the trough; the mix was over packed into
the trough and tightly covered with plastic plate.
The experimental groups containing total of forty
specimens were prepared by adding 0.342 g of
CHX diacetate monohydrate (Fig 5), which is commercially available as a solid powder, to 30g
glass ionomer powder and for 11 gram of RM GIC
0.11g of CHX was incorporated. Within 60 seconds
after the end of mixing, the cements were packed
into the split molds and covered with the plastic
plates. One hour later, the specimens were removed from the mold and stored at room temperature in
artificial saliva (wet mouth – synthetic saliva with
pH of 6.43± 0.26 consisting of 0.8g NaCl, 2.4g KCl,
1.5g NaH2 PO4, 0.1g Na2S and 2 g CO[NH2]2)
for 24 hours.

Flexural strength of the specimens was determined
using three point bending test in a universal testing
machine. A load was applied in the center of the
specimens at the crosshead speed of 0.5 mm/min.
The mounting apparatus was mounted parallel
with the supporting beams 20 mm apart (Fig 14).
The specimens were loaded until the first sound
of a crack was detected. The flexural strength
values of each specimen were calculated with the
following formula:
F=3PfL/2WH2
Where Pf - measured maximum load at the time
of specimen fracture,
L - distance between the supports on the tension
surface (20mm),
W - mean specimen width,
H - mean height of specimen between the tension
and compression surfaces.
Compressive strength of the samples was
determined using universal testing machine
under a crosshead speed of 0.5 mm/min until the
specimen fractures (Fig 15).
Table 1- Comparison of mean compressive strength
of GIC and GIC incorporated with CHX using
independent t test.
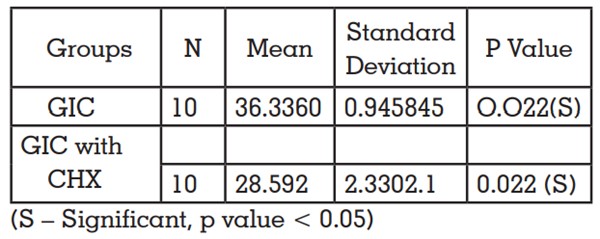
Table 2- Comparison of mean compressive strength
of RMGIC and RMGIC with incorporated CHX using
independent t test.
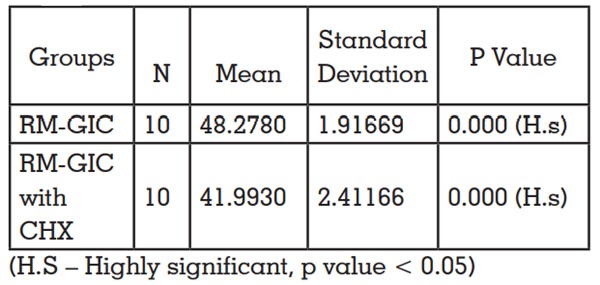
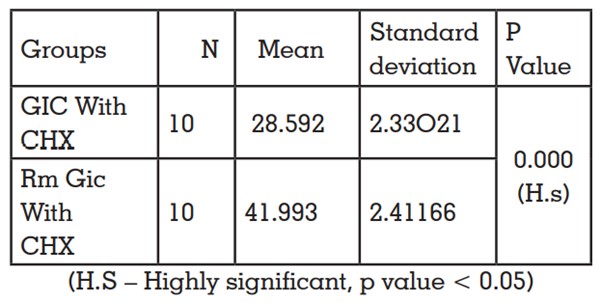
Table 3- Comparison of mean compressive strength of
GIC with incorporated CHX and RMGIC incorporated
with CHX using independent t test.
Results will be recorded in Megapascals.
C = F/πr2
Where F - measured maximum load at the time
of specimen fracture,
r - radius.
Morphological evaluation for fractured
experimental group surfaces was done using
scanning electron microscopy (Fig 16).

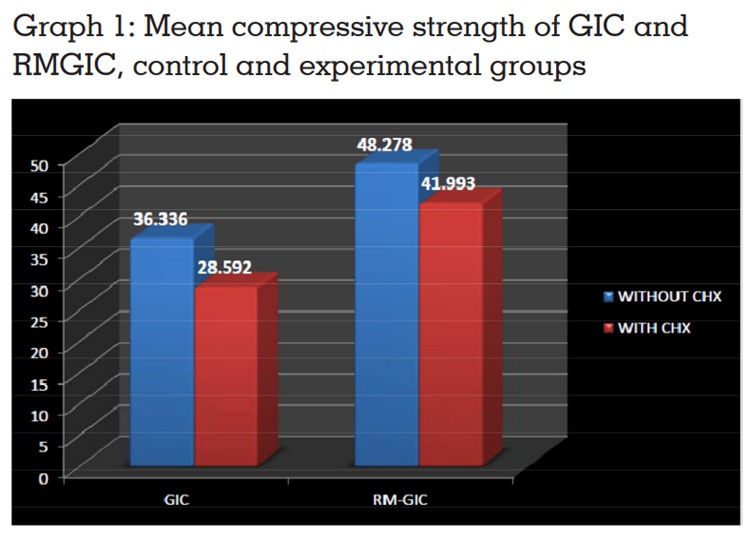
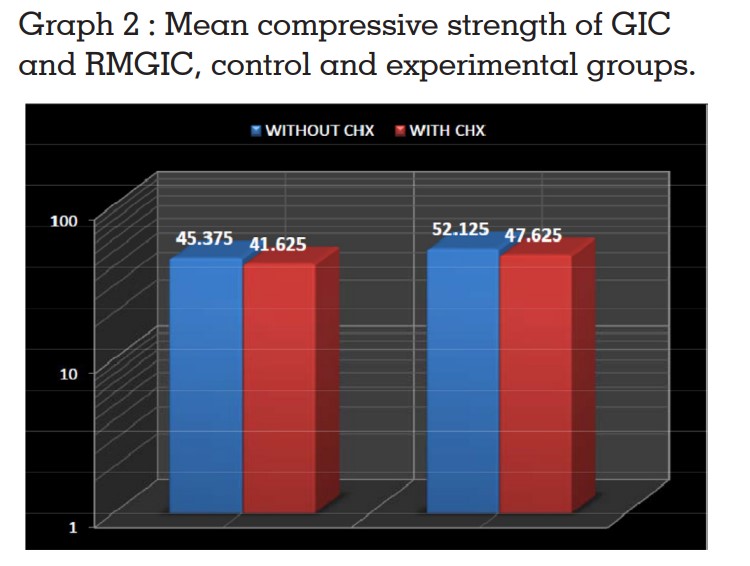
Compressive strength of GIC and GIC incorporated
with 1% CHX was compared, the result obtained
are shown in Table 1. On adding 1% CHX to GIC
resulted in significant decrease in compressive
strength (p<0.05). Compressive strength of
RMGIC and RMGIC incorporated with 1% CHX
was compared. Highest compressive strength
was shown by RMGIC but the strength values
decreased significantly by adding 1% CHX as seen
in Table 2. Compressive strength of RMGIC mixed
with 1% CHX was higher than GIC experimental
group according to independent t-test. Least
strength was shown by GIC mixed with 1% CHX
as shown in Table 3 and is represented in Graph 1.
The effect of incorporation of 1% CHX to
conventional GIC and RMGIC on flexural strength
was compared. In comparison with the control
adding 1% CHX for both GIC and RMGIC resulted in significantly decreased values when analyzed
with independent t test (p < 0.05).Flexural strength
of RMGIC with 1% CHX was higher than that of
GIC with 1% CHX. Highest flexural strength was
shown by RMGIC < RMGIC mixed with 1% CHX
< GIC < GIC mixed with 1% CHX when analyzed
with independent t test (p<0.05).
Morphological evaluation of fracture surfaces
of both the experimental groups were evaluated
by SEM study (Fig 17) shows the SEM image of
fracture surface of GIC with 1% CHX at X1600
magnification. Different sizes of glass particles
which were loosely bonded to the matrix were seen.
Fig 18 shows the SEM image of fracture surface
of RMGIC with 1% CHX at X1000 magnification.
The fracture surface of the RMGIC contained many
small glass particles dispersed in the polymer
matrix. Unlike GIC, the fracture surface of RMGIC
exhibited a more tightly integrated glass particle–
polymer matrix surface and less exposed glass
particles. In addition, large fractured fragments
of the resin constituent were observed in the RM
GICs, compared with the GICs.
Glass ionomer cements (GICs) were introduced in
dental practice in 1972 by Wilson, Kent. The powder
component contains aluminum-fluorosilicate glass that dissolves upon interaction with polyacrylic
acid in the liquid component. The reaction releases
calcium and aluminum ions that interact with the
carboxylic acid groups. Calcium ions present in
the hydroxyapatite of dental hard tissues, enamel
and dentin, react with the carboxylic acid of GICs,
creating a chemical bond between the cement
and the tooth structure.
GICs are widely used in dentistry for its advantages
of potential to inhibit caries because of fluoride
release, adhesion to tooth tissue, reduced marginal
leakage due to thermal changes in the oral
environment since coefficient of thermal expansion
of glass ionomer cement is similar to that of enamel
and dentin. Resin modified glass ionomer cements
(RMGICs) were introduced to provide a material
with improved mechanical properties and the
light cure facility.16
The most commonly used strength value to
characterize dental cements is compressive
strength. It is the ultimate strength to withstand
compression stress mainly for hard brittle
materials.12 However, such materials typically
fail in flexure rather than in compression, and
in recognition of this, there has been some work
in recent years to characterize them in terms of
biaxial flexure strength.22 In order to improve the antibacterial characteristics
of GIC, chlorhexidine (CHX) in the form of
chlorhexidine diacetate powder has been added
to it.22 CHX has also been seen to have long-term
antibacterial properties because of its unique
ability to bind to hydroxy apatite, whereby, a
gradual release creates a bacteriostatic milleu
over a prolonged period of time.4
Addition of CHX
in liquid form results in decreased properties due
to more rapid leaching of CHX in liquid form than
in powder form and CHX diacetate is preferable
to use, as it is a more stable material, not prone to
decomposition, can be easily blended with glass
ionomer powder.22 Therefore in the present study
CHX was added to CGIC in powder form.
In the present study specimens were stored for
a day because a minimum of 24 hr storage is
required for the maturation of GIC, where calcium
ions linked to carboxyl groups of polyacrylic acid
chains are replaced by aluminum ions, and also
according to a study done by Cattani-Lorente
MA et al10.
In the present study incorporation of 1% CHX for
both CGIC and RMGIC resulted in significant
decrease in compressive and flexural strength
properties. The microstructure of GICs is formed
as a result of the acid–base reaction between
the proton donating acidic liquid and proton
accepting basic powder resulting in filler glass
particles distributed within a salt-like hydrogel.25
The compressive strength of the cement arises
from the reinforcing glass filler particles, which
resists compressive forces on loading rather than
the weak matrix.25 In experimental groups RMGIC
had significantly higher values than CGIC, this is
consistent with the study done by Xie D et al9
and
Mallmann A et al.19 This is due to the inclusion of
resinous polymers that present higher mechanical
strength.
Limitations of the study include not considering
the change in temperature and pH which may
occur in oral cavity during consumption of various
beverages. The artificial saliva used did not consist of enzymes and plaque biofilm that is present in
a real life scenario. The result may not be same
to other commercially available GICs due to
difference in the filler size and can be evaluated
for change in strength by adding CHX for other
commercially available materials. Clinically it’s
tedious to keep weighing CHX and cement powder
for proper water powder ratio for each patient. This
can be solved by adding predetermined amount of
CHX to the whole bottle of cement, but the storage
stability of such cements is unknown.
Within the limitations of the study it could be concluded that-