

Aim: The aim of the study was to evaluate the
biocompatibility of denture soft liners using a
fibroblast cell line.
Materials and methods: The effects of two denture
liners (acrylic-based GC Soft Liner and silicone-based GC Reline Soft) on L929 fibroblast cell
lines were investigated. Eluates from the material
specimens were applied directly on the cells and
cytotoxicity of specimen eluates and cell viability
were evaluated by MTT assay and changes in cell
morphology were evaluated by direct contact assay
and inverted phase contrast microscopy. Controls
were cells in culture medium without eluates or
specimens.
Results: GC Soft Liner(acrylic-based soft liner)
showed lower cell viability and more cytotoxicity
than GC Reline Soft (silicone-based soft liner).
Conclusions: Silicone based denture soft liners are
comparatively non cytotoxic to fibroblasts.
The denture soft liners act as a cushion between
the denture base and residual ridge. They are
often used to provide better fit and comfort for
patients who cannot tolerate conventional hard
denture bases because of excessive residual
ridge resorption, bruxism, xerostomia, and fragile
supporting mucosa1
. They have been developed to
help patients when their oral mucosa is damaged
or affected due to ill-fitting dentures or post-implant surgery2
. These soft liners may release
components as residual monomers, plasticizers,
degradation products3,4 and alcohol5,6. Although
reports have indicated that these materials leach
monomers and other components that do affect
their biocompatibility, there is a little information
on what cell molecules may be implicated in these
materials. Only a few studies evaluated the effects
of direct contact between cells and soft liners7–10.
Therefore, the aim of this study was to evaluate
the biocompatibility of denture soft liners using
a fibroblast cell line.
Materials
The materials selected for this study, types,
manufacturers, powder/liquid ratio, compositions and polymerization/gelation time are presented
in Table 1.
Sample preparation
The specimens (discs of diameter 10 mm ×
thickness 1 mm) of each material were prepared
under aseptic conditions. The materials were mixed
according to the manufacturers’ instructions and
inserted into metal molds; pressure was applied
until the reaction was complete. Samples were
exposed in UV irradiation for 30 minutes and were
directly taken for the analysis.
Cell Culture
For biological evaluation, L929 (Mouse Fibroblast)
cells were procured from the National Centre for
Cell Science, Pune, India. The cells were grown
in DMEM supplemented with 10% FBS and
containing the antibiotics penicillin, streptomycin
and amphotericin B (5000 units) in a humidified
incubator at 5% CO2 at 37 ± 0.20C. The cells were
regularly monitored by phase contrast inverted light microscopy. The medium was changed once
in three days. The confluent monolayer was sub-cultured and maintained for further studies11
Evaluation of the toxicity of specimen eluates by
MTT assay
The cytotoxicity of the specimens was evaluated
as per ISO10993-5 on L929 (Mouse Fibroblast)
cell culture. The cells were seeded onto a 48 well
plate and incubated. After attaining confluency, the
sterile specimens were added to the cell seeded
plate. Culture medium without test specimens was
also incubated under the same conditions and
served as control. The percentage of the surviving
fibroblast cells were quantified by the MTT assay
and the morphological changes of the cells were
monitored by phase contrast microscopy12.
MTT assay is carried out to measure mitochondrial
cellular metabolism (viability) and number of
viable cells. MTT assay is based on the capability
of metabolically active fibroblast cells to reduce
the yellow water-soluble tetrazolium salt (MTT) to purple formazan crystals using the mitochondrial
enzyme succinate dehydrogenase (SDH). The
intensity of purple colour so formed is proportional
to the number of viable cells.
Following the experiment, the culture was washed
with 1 x PBS and then 200 µl MTT solution per ml
culture (MTT 5 mg/ml dissolved in PBS and filtered
through a 0.2 µm filter before use) were added. The
whole content was again incubated at 37oC for
3h and 300 µl DMSO were added to each culture
well. The whole content was incubated at room
temperature for 30 min until all cells were lysed
and a homogenous colour was obtained. The
solution was centrifuged for 2 min to sediment cell
debris. The optical density (OD) was measured
spectro-photometrically at 540 nm. Cells treated
with MTT solution without the sample was used as
control. The % viability was calculated as follows:
% Viability = (OD of test)/ (OD of control) X 100
Direct Contact Method
The cytotoxicity of specimens under the direct
contact of cell was determined by direct contact
assay. L929 (Mouse Fibroblast) cells (1x104 cells/m)
were seeded on to a 48 well plate and allowed
to proliferate for 24 h to form a sub-confluent
layer. Then the specimens were placed over the
monolayer and allowed to proliferate in a CO2
incubator. After 24 hours of incubation, cells were
evaluated for changes in morphology with respect
to a control (cells grown without specimens) under
inverted phase contrast microscope (Olympus
CKX41) attached with an imaging camera. The
images were captured using imaging software
Optika vision-pro[13-15].
Statistical Analysis
All statistical analyses were performed with SPSS
for Windows (release 17.0, SPSS Inc., Chicago,
IL, USA). Data from MTT tests (with eluates and
direct contact) were analyzed by two-way ANOVA
followed by Tukey’s test, with P ≤ 0.05.
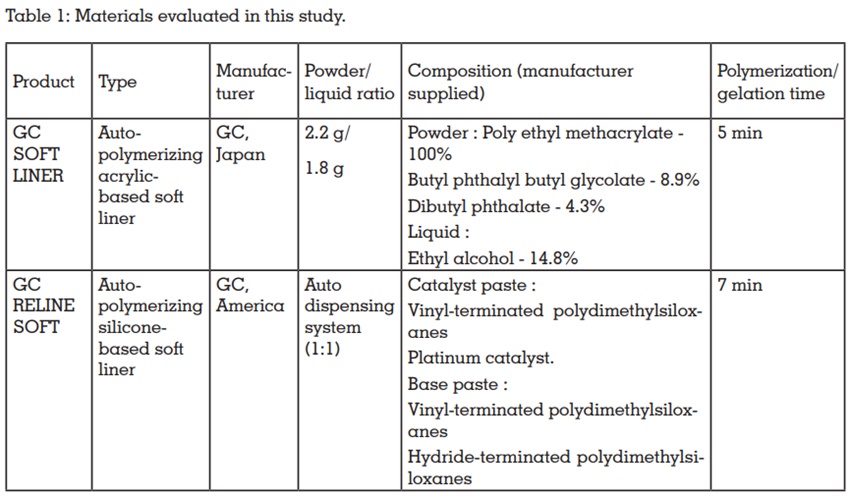
Mean optical density value and percentages of cell
viability relative to controls, obtained in the MTT
assay, are shown in Table 2. For L929 fibroblast
cells, exposure to the 24 hour eluates from GC
Soft Liner (acrylic-based soft liner) resulted in
significantly lower percentages of cell viability
than those obtained with the 24 hour eluates from
GC Reline Soft (silicone-based soft liner).
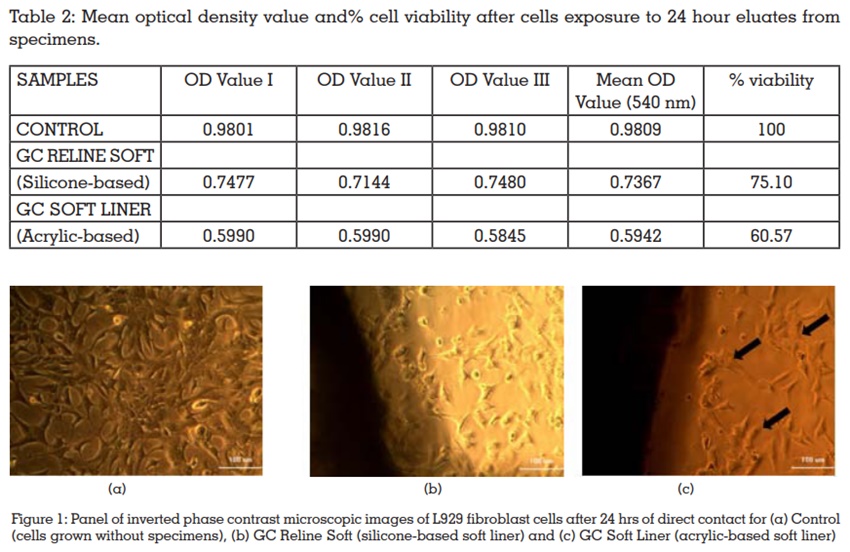
Figure 1 show the inverted phase contrast
microscopic images of L929 fibroblast cells for
controls (cells grown without specimens) and
experimental conditions (cells grown in direct
contact with the soft liner specimens). As shown
in Figures 1(a), control cells displayed their
characteristic spindle-shaped morphology and
undergoing mitosis. GC Reline Soft (silicone-based
soft liner) resulted in a significantly less number of
necrotic cells after 24 hrs of direct contact with the
L929 fibroblast cells [Figure 1(b)] when compared
to GC Soft Liner (acrylic-based soft liner) [Figure
1(c)] but more when compared to control.
The term “cytotoxicity” is used to describe the
cascade of molecular events that interfere with
macromolecular synthesis, causing unequivocal
cellular, functional, and structural damage16. L929
fibroblasts are recommended by ISO 10993-5 for
cytotoxicity tests17. The L929 fibroblast cell lines
used in this study are sensitive to dental monomers
and plasticizers that can be released from polymer
materials.
The results from MTT assay showed that the GC
Soft Liner (acrylic-based soft liner) was more cytotoxic to L929 fibroblast cells compared to GC
Reline Soft (silicone-based soft liner). The absence
of significant reductions in cell viability after
exposure to eluates from the materials does not
exclude the possibility of damage to delicate cell
structures. Thus, in the present study, microscopic
analysis of cellular morphology was performed.
Inverted phase contrast microscopic images were
used to assess changes in cell morphology induced
by direct contact of L929 fibroblast cells with the
soft liner specimens. For direct contact (24 hr time
period), the silicone-based soft liner GC Reline
Soft was less cytotoxic to the L929 fibroblast cells,
while the acrylic-based soft liner GC Soft Liner
exerted greater effects.
Silicone-based soft liners are similar to
polyvinylsiloxane-based impression materials1
and polymerize by an addition reaction with no
by-products, such as alcohol. It has been found
that, although components such as monomers
and phthalic plasticizers were released by soft
liners, the silicone-based materials were generally
more stable, releasing smaller quantities than the
acrylic-based softliners3,4. Thus, it can be supposed
that GC Reline Soft exerted less-pronounced
cytotoxic effects on the L929 fibroblast cell lines
due to a lower release of residual components.
The materials evaluated also contain plasticizers
such as dibutyl phthalate (DBP) in GC Soft Liner
(acrylic-based soft liner). The toxicity of phthalates
is caused by their metabolite methoxyacetic acid
(MAA), through mechanisms that involve ROS
generation and DNA and mitochondrial damage18.
The exposure of L929 fibroblast cells to DBP for
24 h19 led to a reduction in DNA synthesis, cell
metabolism, and viability. Thus, it is likely that
the cytotoxic effects observed here for GC Soft
Liner (acrylic-based soft liner) are related, at
least in part, to the presence of plasticizers in its
composition.
Other studies also found that the direct contact
between different cells and denture liners and
tissue conditioners resulted in cytotoxic effects, such as zones of growth inhibition, cell lysis7
, and
reduced cell viability20. Kruni’c et al. observed
lower cytotoxicity for the silicone-based soft liners,
compared with the acrylic-based materials21. Park
et al. evaluated the cytotoxicity of short-term-use
soft liners after repeated elution using the agar
overlay method. Although cytotoxicity decreased
after repeated elution, they recommended these
materials to be used within a limited time22.
Ozdemir et al.16 found that a vinyl polysiloxane
material exhibited heightened cytotoxic effect
after 96 h of incubation. Munksgaard23 showed
that the leaching of phthalate during the first
day of use exceed tolerable daily intake by 11–32
times for different materials and this may cause
undesirable biological effects. In an another study,
Munksgaard24 reported that an esterase activity,
equivalent to that in saliva in the immersion
medium for soft lining materials increased the
rate of diffusion of plasticizer from the materials.
Mutluay and Ruyter25 pointed that, although not
reported previously for vinyl polysiloxanes, allergic
reactions should be always kept in mind because
of applying fresh uncured polymer directly to the
mucosa. Dahl et al.,26 investigated the in vitro
cytotoxicity of denture relining materials using cell
culture tests and a test for irritation mechanisms.
They stated that five of the tested materials were
slightly or moderately cytotoxic in the contact test.
They also reported that nine of the eleven products
showed cytotoxic response in the MTT test using
extracts of the test specimens.
Although the results obtained from in vitro studies
cannot be directly extrapolated to clinical situations,
it is important to consider whether the changes
observed in the present investigation are cumulative
and become increasingly pronounced with time.
This could make the cells more susceptible to
subsequent challenges, such as direct contact with
newly applied soft liner materials. It is important to
note that, due to a progressive loss of plasticizers
and alcohol, the soft liners need to be replaced at
regular intervals. Although these replacements will
prevent trauma and colonization of the material by microorganisms, they are performed directly
in the mouth, increasing the exposure of tissues
to the leached components, which, even in low
concentrations, can lead to chronic adverse
effects on the oral mucosa. Even if not causing
acute cytotoxicity, the continuous release of such
substances may compromise tissue homeostasis
and repair, which are particularly important given
that some softliner materials are applied over
inflamed supporting tissues and during the healing
phase in immediate dentures or dental implants27.
Within the limitations of this study, the following conclusions were drawn:
All these findings are importance because the
soft liners are often applied on areas of ulcerated
tissue or after surgery, where both macrophages
and fibroblasts play important roles in the healing
process27.