

Purpose: The purpose of this paper is to analyse the current understanding of clinical assessments and factors that determine the success and failure of osseointegrated dental implants.
Study Selection: This is a narrative review performed through scientific articles published between the year 2000 and 2017, indexed in MEDLINE and pubmed databases. The study selected articles that focused on osseointegration around the implant body.
Results: The Implant –Tissue interface is an extremely dynamic region of interaction. The process of osseointegration involves an initial interlocking between alveolar bone and the implant body and later biological fixation through continuous bone apposition and remodelling towards the implant. The process itself is quite complex and there are many factors that influence the formation and maintenance of bone at the implant surface.
Conclusions: This narrative review explores the extent of current knowledge of osseointegration and aims to consolidate this knowledge and highlight the most pertinent questions relating to this area.
Osseointegration is a phenomenon where intimate contact between bone and biomaterials occurs at the optical microscopy level, enabling dental implants to replace load bearing tooth organs restore their form and intraoral function4. A thorough and complete understanding of what happens at the bone and implant surface is important and thereby enabling a treatment plan which conforms to the standards and gives a better clinical predictability2.
The term osseointegration was first used by prof I.P Branemark, in 1950, during an examination of microcirculation of bone and wound healing through means of vital microscopy, and accidentally discovered the process of osseointegration and a new dimension in the field of implantology.
The healing of an osseous wound around a dental implant is a coordinated and sequentially organised repair mechanism. The process takes place by the communication between different types of cells. This sequence of cell communication is explained as the four phases of osseointegration.
Phase 1- Hemostasis
Phase 2- Inflammatory phase
Phase 3- Proliferative phase
Phase 4- Remodelling phase
Hemostasis
Hemostasis or exudative phase begins with the surgical drilling by the dental implant drill followed by the placement of implant. The duration of this phase is minutes to hours. The mechanical crushing of the bone matrix liberates bone matrix protein, growth factors and differentiation factors into the implant site. Injured blood vessels create a polymerization of fibrinogen with the help of thrombin. Bioactive molecules such as thrombin, ADP, collagen, fibrinogen and thrombospondin are generated. Thromboxane released from platelets plays a critical role initial vasoconstriction. The release of cytokines from the degranulated platelets is the beginning of inflammatory phase.
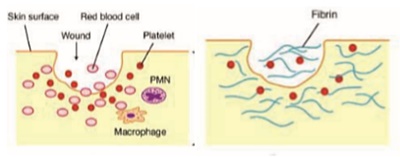
Inflammatory Phase
Inflammatory phase begins approximately after 10 minutes and lasts for first few days after surgery. This phase begins with degranulation of platelets and release of growth factors like transforming growth factor (TGF-B), platelet derived growth factor (PDGF), Fibroblast growth factor (FGF), Bradykinin etc1. Bradykinin increases the vascular permeability and endothelial cells in the blood vessel wall promote the attachment of pmns/leucocytes. The pmns invade the blood clot by amoeboid migration, squeezing through little gaps in the walls of blood vessels with the help of proteases and finally enter the wound site. Leucocytes/ pmns then chemotactically navigate through the wound along the molecular concentration gradient. The molecules include bacterial proteins, fibrinopeptides and proinflammatory interleukins. On arrival, they kill the bacteria through the release of reactive oxygen species. Pmns also release highly digestive enzymes such as collagenase and elastase.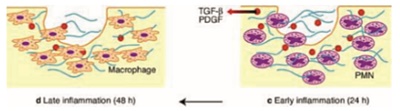
The wound then proceeds to a stage of healing process. The tissue debris are biochemically degraded, and underwent phagocytosis by macrophages. The macrophages synthesis proinflammatory cytokines and proteases using antigenic inhibitors for digesting proteases, macrophages stops the tissue degradation started by pmns. This will in turn produce growth factors by matrix proteins and proteoglycans.
Proliferative phase
The transition into proloferative phase is characterized by the formation of new extra cellular matrix and angiogenesis. Messenger substance like VEGF, PEGF, FGF stimulates fibroblasts and helps angiogenesis. Fibroblasts appear on the 3rd or 4th day. They migrate into the wound by amoeboid movements. They synthesis the protective and stabilising components of extracellular matrix such as collagen, elastin and proteoglycans.
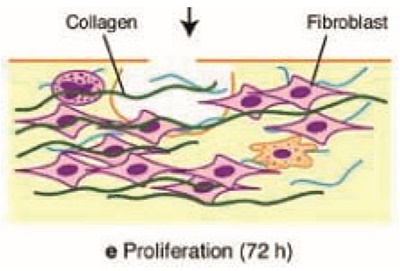
The low oxygen concentration in the wound affects both macrophages and endothelial cells and stimulates them to create the intercellular transcription factor, hypoxia inducible factor. VEGF influence perivascular cells. They migrate into VEGF gradient, into areas of low partial oxygen pressure. Here they form new blood vessels, which integrate into existing network.
Angiogenesis restores the oxygen supply, which is a prerequisite for osteogenesis. Activated osteoclasts attach themselves to the fracture areas of residual bone, resorbing it and creating space for bone healing1. However, it will influence the primary stability of implants. Osteoclasts dissolves the bone by the action of hcl and proteases and release BMP, TGF-B, PDGF from bone matrix, which in turn initiates the formation of new bone. Perivascular cells migrate into implant surface and differentiate into new osteoblasts under influence of bmps. Osteoblasts then forms an organic matrix, along with calcium phosphate. Towards the end of 2 weeks woven bone is formed at the implant surfaces, this will create a secondary stabilisation. The formation of woven bone concludes the proliferative stages of wound healing.
Remodelling phase
The initial bone formed, ie; woven bone is grown parallel to the implant surface. On remodelling phase, parallel bone formed is structured perpendicular to the peak of implant threads. Organisation of the bone becomes more trabecular. This process happened by the coupling action of osteoclasts and osteoblasts.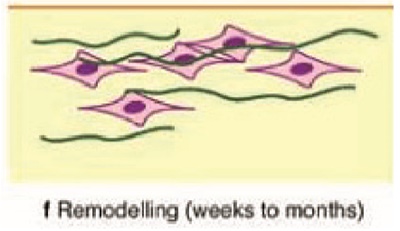
Osteoclasts activated by osteoblast messenger RANKL, resorbs the woven bone and the osteoblasts lay down highly organised lamellar bone. This simultaneous action of osteoblasts and osteoclasts are coordinated by osteocyte and its messenger sclerostin. In the final state, the newly formed unit, containing a central blood vessel called osteone or Haversian system.
Successful osseointegration from the clinical stand point is a measure of implant stability, which occur after implant integration. Alberkstsson et al referred to six factors determine the success of osseointegration, implant biocompatibility, design characteristics, implant surface, state of host bed, surgical technique, implant loading condition.
One of the most important aspects about achieving osseointegration clinically is implant primary stability. Initial or primary stability is the mechanical interlocking between the bone and the implant where there exists no biologic interplay13. The mechanical interlocking is influenzed by the implant geometry and topography at different levels, as well as the implant osteotomy protocols,which all regulate the strain applied to the hard tissue18. The higher insertion torque of the implant is intuitively and fallaciously perceived as higher primary stability,which has been clinically regarded as an indication for procedures such as immediate loading15. However, in reality, the stability of the implant would decrease beyond the yield strain of the bone due to excessive micro crack formation and compression necrosis, which both phenomena trigger bone remodelling12. Compression necrosis occurs when the hard tissue around the implant is faced with excessive strain,where the circulation of the capillaries and nerves is severely damaged19.
The surgical drilling speed proportionally influences heat generation to the surrounding bone14. Research has indicated that an overheat exceeding 47°C for 1 min would provoke an irreversible thermal injury to the bone with osteoclasts activation due to local surgical instrumentation damage or osteocyte death20. Yeniyol et al have demonstrated that higher degree of osseointegration for implants placed in sites prepared under low-speed drilling. A recent experimental study has shown that higher osseointegration levels and lower degree of bone dieback for drilling less than 400 rpm17. Bone cell survival is very susceptible to heat. Eriksson has demonstrated that in rabbit, bone temperature as low as 30°c above normal (40°C), can cause bone cell necrosis. Therefore, a conscious effort is made to control temperature elevation every time a rotory instrument is placed in contact with bone. Atleast 50ml/min of cooled irrigation.
The original Branemark surgical approach should be used to maintain aseptic surgical protocol. Normal skin flora, including staphylococcus epidermis and staphylococcus aureus are able to adhere to implant biomaterials. Abacterial smear layer may develop, which will be difficult for the body to remove by phagocytosis or bone cell activity. Antimicrobial mouth rinses significantly reduce bacterial count in the saliva for more than 4 hours. Povidone iodine alcohol solution have antibacterial properties used in preoperative site disinfection. Although a sterile technique is not possible within the mouth, scrubbing around and within the mouth with these agents reduce the risk of a bacterial smear layer by inadvertent contamination by direct contact with the implant or indirectly via a glove or instrument21.
Attempted to concise the process of osseointegration through the four phases of tissue healing. Factors affecting the process of osseointegration provides the clinicians, information regarding the importance of surgical protocol. Experiencing implant osseointegration as a biological process may provide the clinician new targets to improve the therapy with dental implants.